Solution to problem no. 2
(b) is correct, entropy of the cool block increases and entropy of the hot block decreases. The entropy changes cannot have the same direction because the processes in each block are opposed. Conclusion: hot objects have higher entropy than cold objects, if everything else is the same.
A closer look at the entropy changes reveals that entropy must decrease less in the hot body than it increases in the cold. Note that absolute temperature change in both blocks are equal. It follows that the same amount of heat added to a hot body increases its entropy less than when added to a cold body - everything else being held constant.
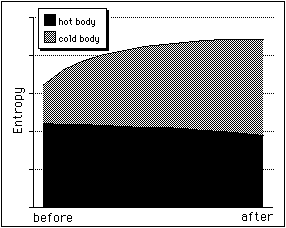
The picture shows the entropy changes (arbitrary units). While the entropy of the hot body decreases, the entropy of the cold body increases even more so that overall entropy increases. Please note that local decrease of entropy is possible, as the case of the hot body shows.
The system in consideration, the hot block by itself and the cold block by itself, respectively, are both closed systems. Matter does not cross the block boundaries, but heat does. As opposed to problem No. 1, this cannot be neglected since we are studying the effects of that heat transfer.
back to article