T = 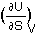
Remember that
temperature is the ratio of internal energy (U) change by entropy (S) change,
T =
As long as the water is boiling, temperature stays constant, and so does the slope in the U/S-diagram. So you have a straight line:
Fig. 1
During phase transition, the internal energy of a system changes linearly with entropy.