You can always take readings of a measuring device like a thermometer. But they don't always have any meaning. How to make sure they do, and how theory can help achieving this very practical aim is discussed in the following. Temperature does not depend on how it is measured, but not every quantity shares this property; what are the conditions for physical quantities to be independend on the observer and the specific procedures she prefers? What is temperature and why can we be confident that it is a real property of systems?
The laws of thermodynamics are rather chary of temperature; the Zeroth Law merely states:
there is a quantity that is equal among bodies at thermal equilibrium.
This is more than nitpicking pedantry but also important in practice, since you expect several readings of the same thermometer to give the same number and, even more, several thermometers to give the same temperature. If there is no equilibrium between thermometer and the body, thermodynamics says that you cannot expect these statements to be valid and hence, the meaning of the term "temperature" is questionnable.
The reading of a thermometer shone at by the sun gives more or less the temperature of the thermometer itself - but this is usually not what you want to know. This temperature is the result of a dynamic equilibrium: a certain amount heat Q1 flows from the sun (heat source) to the thermometer and an other amount Q2 from the thermometer to the air (heat sink); if the heats are different, the temperature of the thermometer changes. For instance, the thermometer gets hotter if Q1 is larger than Q2 and it gets cooler if Q2 is larger. But Q2, among others, increases with increasing temperature of the thermometer. Thus, at given Q1, eventually both heat flows become equal, and a dynamic equilibrium is reached. Unfortunately, this temperature has nothing to do with the temperature of the surrounding air.
On the other hand, if the thermometer is placed in the shadow, the sun as a heat source to the thermometer is removed and a (static) equilibrium between thermometer and air can be attained eventually. That is, the heat source and the heat sink are identical in this case. Then each thermometer measures the temperature of the air, and if appropriately calibrated, gives the same reading.
If you want your thermometer reading to mean more than the figures that show up when throwing dice, you have to carefully determine
In short: you can always take readings of a thermometer, but they do not always make sense; it is your responsibility to make sure they do. By the way: this applies to every kind of measurement.
Didn't imagine measurement of temperature being that intricate, huh?
Of course they don't.
So does this mean that temperature depends on what substance you take and what property you choose to determine it? Yes and no:
No - the temperature itself does not depend on the thermometric substance. It must not, if temperature is to mean more than just a heuristic concept.
Yes - in the sense that the reading of temperature depends on the method you use to get it. This means that a thermometer only gives an estimation of temperature which may be biased depending on what method you use. Hence, for accurate measurements, any thermometric device has to be calibrated with the thermodynamic temperature scale.
V
= V0 * (1 +
*
)
= 1/273,
T =
+ T0.
VT = V0 * (1 + (T - T0)/T0
VT = V0*T / T0
He could do this after the efficiency of a carnot cycle has been discovered to depend only on the temperature of the heat source and the one of the heat sink (Sadi Carnot, 1824). If this holds, temperature can be defined as the quantity that determines the efficiency of a Carnot cycle. A closer look at the Carnot cycle reveals that its efficiency is the maximum work you can get out of a given amount of heat. Remember the definitions of heat and work:
The First Law is the well-known energy conservation law. It states that every change of internal energy of a system must be due to energy transferred to it from the outside. Energy can be transferred to or from a system as heat or as work.
dU
= 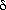 Q
+
Q
+ 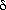 W
W (eqn. 1)
where
Energy is transferred as work, if a process does not change the entropy of the system, and it is transferred as heat, if entropy of the system is changed. These statements define heat and work, respectively.
The Second Law comes in two flavours:
Of course the two "flavours" are one; they are logically equivalent. The Kelvin statement is related to the efficiency of a Carnot cycle: it denies the existence of a heat engine with efficiency equal to unity. We have seen before (in the note about the carnot cycle) that this leads to the conclusion that, for a reversible Carnot process,
Q/T = constant or
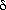 Q/T = 0
Q/T = 0
Thus for reversible processes Q/T is conserved. Mathematically, it is a total differential, called the entropy dS. Hence, for reversible processes, total entropy is conserved,
dS = 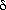 Qrev/T
Qrev/T
If the process is not reversible, the heat engine loses energy by friction, hence
Qhi/Thi > Qlo/Tlo
Qhi/Thi - Qlo/Tlo > 0
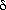 Q/T > 0
Q/T > 0
dS > 0
To put this bunch of formulas into words:
dS = 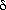 Qrev/T
Qrev/T (eqn. 2)
as the Second Law.
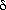 Q = TdS
Q = TdS
dU = TdS - pdV,
where we are considering only PV work. Since the internal energy U is a total differential:
dU =
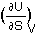 +
+ 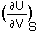
Thus by comparison:
T = 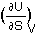 ;
;
- p = 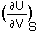
This can serve as the definition of temperature we have been looking for. Let us see how it works.
If a given amount of heat is transferred to a system then its iternal energy is rised, and so does its entropy. This is depicted in the following diagram
Picture 1The image shows how internal energy changes upon increase of entropy; if this suggests to you that it must be possible to take control of the entropy of a system, then you are on the right path. Keep in mind that the changes in the system we are discussing here are caused by the addition of heat, and this, by definition, is the transfer of energy while changing entropy. Thus, by heating or cooling (among other), you can control the entropy of a system.
In this picture, the slope at a given entropy indicates how much the internal energy goes up for an infinitesimal change of entropy. This ratio is the temperature. Thus, from the First and Second Law combined it follows that
temperature is the ratio of internal energy (U) change by entropy (S) change,
T =
The diagram indicates that at high entropy, the slope is steeper than at low entropy. That means, since the slope is temperature, that temperature goes up from the left to the right. This corresponds to the fact that at high temperature, adding a given amount of heat to a system, raises its entropy less than does the same amount of heat at a low temperature. As worked out in the "historical approach to entropy"article (not yet available), this is essential to make heat engines work.
As an exercise, you may try to figure out how this diagram looks like for a system undergoing a phase transition. To be specific, imagine a pot of boiling water. In spite of heating, temperature stays constant, but the water changes its phase from liquid to gaseous. What would the diagram look like? Click here for a solution



Last update Mittwoch, 8. April 2009